|
In the excitement of conceiving your killer ap, you instinctively want to “build a prototype” so that you can show it around to possible investors and partners. Or you want to build a prototype to see if your idea will work. You will eventually have to do both of those activities if your product will have a physical embodiment, (as opposed to software or pharma for example, where the analogous “prototype” would likely be a written description, and not need a physical model to be constructed). A note for developers working in the digital device realm - most of this article will also be applicable to your activities; one important additional one being the need for additional fail-safe considerations for the patient. In your software or firmware controlled device under development, it could take a skilled "forensic" engineer tease out all the possible failure modes. The Three Pillars of Risk To succeed at your product development project, you will have to overcome the three pillars of risk:
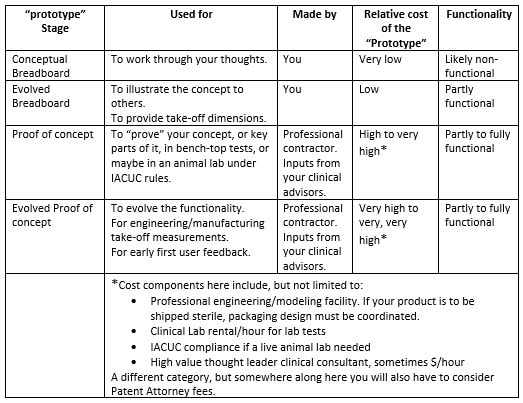
In discussion with a leading Product Development associate, we are reminded how complex each of these areas are, with Technology often being "easier" for a particular project than the latter two. Their advice? Focus on quantifying Market and Regulatory risk before getting too far into product development. This can be contrary to the instinct of inventors and engineers who typically focus on Technology issues while ignoring the many lurking deal breakers in the Regulatory and Marketing arenas. Take a Step Back So, before you contract with a professional to engineer and build your prototype, you will likely be better served by taking a step back and considering what you really need at this stage, and what the returns on your dollars invested will be. The goals here being that you want to map out the most efficient use of your starter/seed funds, and also to be able to go to your professional model builder with a clearly defined description/scope of work for your prototype needs. Firstly though, it’s important to understand that there are different kinds of prototypes, built for different purposes. In your application, prototypes might represent the progressing evolution of your thinking. Or they might be just a subsection of the whole, intended for example to help assess your Value Proposition. Or, to enable your eventual professional model builder, or your IP counsel, to better understand some aspect as they prepare their diagrams. What is the purpose (or "Stage" of your proposed prototype?) In this table I illustrate roughly the purposes for which a prototype might be built. Any one of these "stages" could also be a point where, if this is your strategy, you could dress up your idea to sell it, or to seriously look for funding or to look for partner(s). Real-world examples of different Stage prototypes 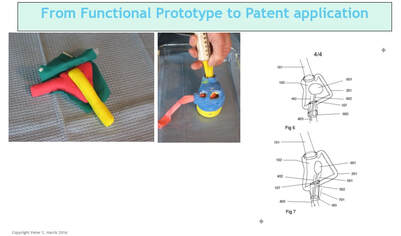 Image 1 shows a real-word prototype, as was used to illustrate its subject concept, and to provide guidance in the preparation of patent drawings and claims. Simple, effective, and necessary. Other prototype versions were built to model functionality.  Image 2 shows a real-world “breadboard” prototype, used to provide take-off dimensions - in this case, for mechanical components. It would be similar for a for a device comprising electronic components such as switches, cables, plugs, processors etc. Electrical and mechanical functionality were demonstrated on a different model. 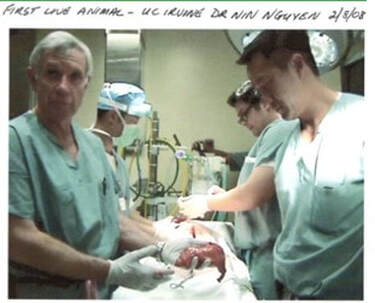 Image 3 shows a real-world “proof of concept”, using a professionally engineered and built prototype, in an animal lab. This requires significant preparation and expense including recruiting and training a qualified surgeon (unless you are one), acquiring a suitable animal model, and renting OR and RR space, and compliance with extensive IRB and IACUC rules governing the care of the the animal subject. This lab was done in order to verify the in vivo functionality of the end effector of the subject device. To be clear – none of the challenges mentioned should deter you – this is simply the Medical Device landscape in which you have chosen to operate.
Suggested next steps Roughly in priority order, and most of which will require some level of funding from your seed funds:
Now you can go back to your Medical Device Modeler Once you have accumulated enough GO flags, you can go back to your Medical Device Modeler and discuss your prototyping needs with confidence and credibility. A Heads Up Don't underestimate early attention and seed funding needed (if you are not already set up and seasoned in this field) for the formal documentation of your R&D efforts on your device concept. It is beyond the scope of this article other than to give you a heads-up to become familiar with the main documentation requirements of the FDA, such as for starters: Design History File; Device Master Record; Device History Record; FDA Design Controls 21 CFR Part 820.30.The FDA can be quite severe in penalizing for transgressions, and my advice is to embrace the guidelines for the good they do and to never try to circumvent them - it is not worth it. So whether you are experienced, or new in this business, now would be a good time to reach out to the experts at BizPush and MedSurgPI for a no-commitment exploratory discussion. Contact us! Comments are closed.
|
AuthorPeter Harris brings a career in medical devices with companies such as Hewlett-Packard and GE Medical, but has found equal challenges and rewards in working at the start-up level and as a self-employed Consultant. ArchivesCategories |

 RSS Feed
RSS Feed

